The Helmet That ‘Resets’ Your Brain
Magnetic stimulation is helping some people with depression—but the $12,000 treatment is also being unleashed in untested ways.
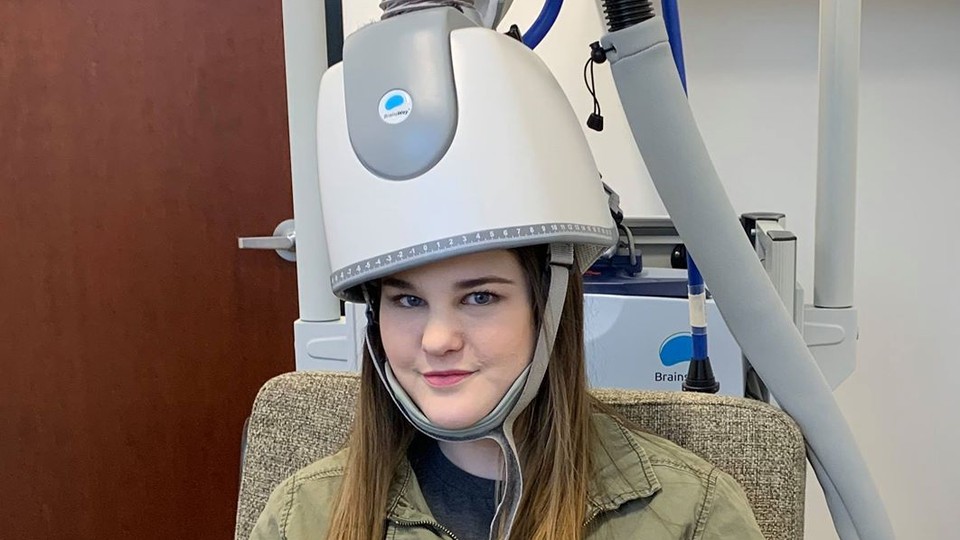
The past two weeks have been frenetic for Bre Hushaw, who is now known to millions of people as the girl in the depression helmet.
Hushaw has been hearing from people all around the world who want to try it, or at least want to know how it works. Her life as a meme began when she agreed to an on-camera interview with the local-news site AZfamily.com for a story headlined “Helmet Approved by FDA to Treat Depression Available in Arizona.” The feel-good tale of Hushaw’s miraculous recovery from severe depression was tossed into the decontextualizing maw of the internet and distilled down to a screenshot of a young woman looking like a listless Stormtrooper.
Jokes poured in. Some of the most popular, each with more than 100,000 likes on Twitter, include: “If u see me with this ugly ass helmet mind ur business.” “Friend: hey everything alright? Me, wearing depression helmet: yeah I’m just tired.” “The depression helmet STAYS ON during sex.”
Hushaw has been tracking the virality, sometimes cringing and sometimes laughing. She replies to as many serious inquiries as she can, while finishing up her senior year at Northern Arizona University before starting a job in marketing. A year ago, she didn’t think she was going to live to graduation. When she was 10 years old, her mother died. Her depression symptoms waxed and waned from then on, and they waxed especially when she heard the gunshots on her campus during a shooting at the school in 2015. She’s tried many medications over the years—14, by her count.
“From age 15 until I was 20, I was extremely suicidal, and I was self-harming,” she told me last week. She recounted multiple related hospitalizations, and a gradual loss of faith in the medical system.
So last year, when Hushaw learned of a helmet that promised to magnetically rewire her brain, she saw this as an obvious yes. The helmet contains magnets that exert energy on the electrical functioning of the brain, a process known as transcranial magnetic stimulation, or TMS. Hushaw went to a clinic and absorbed electrical impulses for 20 minutes every (business) day for six weeks.
Though Hushaw likens the feeling to being “tapped” by a pencil, the chin strap makes it appear as if the helmet is going to blast her with energy. This didn’t help with the jokes. I retweeted the news story with, “After wearing it you feel like a weight has been lifted off you.” That made me feel clever until I actually read the story and saw that Hushaw had said almost exactly the same thing—“I felt like there was a huge blanket that was lifted off my shoulders and I felt completely free”—referring to suicidal depression.
Hushaw is okay with it. Despite the mockery, overall she’s thrilled by the attention that’s been given to the helmet. The image above is a re-creation—she went back to the clinic to take the photo, and she sent it to me. I didn’t ask her to do this. But she is passionate: “I just want to make sure that people are getting help,” she said. “I had a friend commit suicide on my campus, and I just never want that to happen again.”
As she put it multiple times, “It actually, really saved my life.”
The attention that Hushaw’s story received is a testament to how few people know what to make of TMS. Even when I surveyed physician friends about it, several hadn’t heard of it, and no one had seen it used in more than a rare case. It is certainly not woven into typical treatment plans.
Researchers at some academic institutions are taking the technology seriously. Yale has a Repetitive Transcranial Magnetic Stimulation Research Clinic, and the service is offered at Johns Hopkins. Numerous studies have suggested promising clinical uses, including one this week in the journal Neurology. But the mechanisms proposed are vague. TMS may be beneficial in treating addiction, according to a 2017 paper in Nature Neuroscience Reviews, by “influencing neural activity ... throughout the brain.” According to the Mayo Clinic: “Though the biology of why TMS works isn’t completely understood, the stimulation appears to impact how the brain is working, which in turn seems to ease depression symptoms and improve mood.”
Yes, TMS seems to affect how the brain is working. These statements are not abdications of explanatory burden, but come close to the extent of what is known. Serenity Mental Health Centers, the Arizona clinic that provided Hushaw with the electromagnetic treatment, claims that “people with depression often have areas of their brain with decreased activity, and people with [obsessive-compulsive disorder] often have overactive areas of their brain, so TMS stimulates and resets those regions of the brain.”
The notion that the device has dramatic effects on the structure or function of the brain is at odds, though, with the U.S. Food and Drug Administration’s classification. In March, the regulatory agency issued a rule deeming it a Class II medical device, along with electric wheelchairs and pregnancy tests, which means that it is presumed to be safe. Most therapeutic devices that affect human physiology are Class III, which would certainly be the case with anything that “resets the brain.” The FDA argued that a Class II designation would “enhance patients’ access to beneficial innovation, in part by reducing regulatory burdens by placing the device into a lower device class than the automatic Class III assignment.”
The first TMS helmet approved by the FDA, NeuroStar, was for treatment of major depressive disorder, in 2008. Others have been approved since, as the market began to boom. On Tuesday, BrainsWay, the company that made the helmet used by Hushaw, announced its initial public offering. BrainsWay was also approved for obsessive-compulsive disorder in August 2018, and the publicity efforts around this approval were what eventually led to Hushaw’s news fame, according to the marketing director of the clinic that provides the helmet, Serenity Mental Health Centers.
This marketing director, Candise Miller, has her own miraculous personal story of recovery via TMS. “My life is forever changed. I’m a completely different person,” she told me. She asked me to include a link to the clinic’s home page, which features her testimonial but does not mention that she is director of marketing.
Until the FDA’s new classification this year, the agency had reined in marketing of such health claims by requiring premarket approval for TMS devices. Manufacturers had to submit evidence that the devices had no immediately obvious adverse effects and at least a small amount of effectiveness. For instance, the FDA said it based approval of BrainsWay for OCD on a single study of 100 people, which showed improvements in some patients. A control group wore actual TMS helmets that secretly weren’t turned on. Presumably due to a placebo effect, this group also saw an 11 percent decrease in symptoms.
Like most treatments in psychiatry, there is value in showing up, and in believing you are being treated. These and other mechanisms are mysterious, and the effects are unreliable—attesting to the complexity of mental illness, and the many factors that go into causing and treating it.
The basic idea of shocking the system into compliance has deep roots. Since electroconvulsive therapy was introduced almost a century ago, the approach has been shown to be unreliably but sometimes dramatically effective for treatment of severe depression. At least partly due to its barbaric connotations and the uncertainty of the outcomes, electroconvulsive therapy remains one of the most controversial treatments in medicine. It isn’t practiced by most psychiatrists.
The electrical charges delivered by TMS are meant to be more focused, but still very powerful. Inside the helmet, a series of looped wires are connected to capacitors that pass electrical currents through them in bursts. Pulses generate a secondary electric current that alters the electrical fields in the brain, depolarizing neurons and causing them to fire. The scalp and skull do not shield the electrical processes in the brain from such a force any more than a cubicle wall shields your ears from a colleague who is incapable of keeping his phone on silent.
Whether or how TMS would cause a lasting change in brain function is not entirely clear. The concept was introduced in 1985 at the University of Sheffield, in England, as a diagnostic and mapping tool for the motor cortex. The technology can reliably be used to make a person’s legs jerk, but the ostensible aim of the current treatments is to reach beyond transient cortical activity and fundamentally alter the brain’s circuitry. And unlike the invasive neurological procedure of deep-brain stimulation, which has proved useful in treating OCD as well as Parkinson’s disease and other conditions, the helmet doesn’t require any holes in the skull and electrodes planted in the brain.
But TMS’s marketing claims raise questions about how the helmet’s electrical currents could reach the brain’s emotion-driving portions without causing any unwanted cortical activity or serious adverse effects. In electroconvulsive therapy, a person must be anesthetized and made to convulse, and this was always seen as an unfortunate byproduct of the attempt to reset deeper parts of the nervous system. TMS requires no sedation, and only rarely causes seizures. (The sessions are still supposed to be closely monitored by a licensed technician—and the helmet is not supposed to be worn in public, as was implied in most of the jokes that hit the internet last week.)
The only people who claim to know precisely how these helmets treat such complex sociocultural-behavioral conditions as depression and anxiety are the ones selling treatment with the machine, or the machines themselves. As TeeJay Tripp, the medical director of Arizona’s Serenity Mental Health Centers, who treated Hushaw, understands it, TMS activates the prefrontal cortex, which can lead to downstream effects that ultimately affect the amygdala or other deep structures tied to emotion.
The lack of understanding about what might be happening in those deep structures is paired with uncertainty about what parts of the cortex should be stimulated in the first place. The common wisdom among TMS practitioners is that depression occurs in the right side of the brain, and anxiety on the left. Depending on which you have, the energy needs to be focused on one side. But this two-sided model of the brain is not supported by any neuroscience text I can find.
In addition to treatments for depression and anxiety, Serenity Mental Health Centers also offers to provide TMS for “ADD/ADHD, addiction, Alzheimer’s disease, anxiety, autism, bipolar disorder, chronic pain, eating disorders, multiple sclerosis, schizophrenia, stroke rehabilitation, and substance abuse.” The FDA has only approved TMS for depression and OCD, but the approach can still legally be used “off-label.” When asked where in the brain electricity should be applied for these various conditions, Tripp said he bases his treatment on trial and error, along with whatever small studies have been done on any particular condition. Most notably among these uses, he and other practitioners have begun putting the helmets on children with autism.
I asked Tripp whether he was concerned about potential long-term repercussions, or simply about rewiring the wrong area. He cited the fact that the FDA approved the helmet 10 years ago (for use in depression), and no research has yet shown long-term harm. Miller, Serenity’s marketing director, believes that TMS’s overhead so far has prevented it from being more widely known and used; she put the ballpark cost of a BrainsWay device at $200,000. She also contends that uptake has been slow because of “Western medicine’s reliance on pharmaceuticals,” and insurance companies’ unwillingness to pay for it.
More insurance companies are covering the treatment, though. Direct-to-consumer marketing has increased demand in recent years, practitioners tell me, and many plans will cover the $10,000 to $12,000 treatment for people who haven’t responded to trials of medications and therapy.
This is how LeeAnn Tucker afforded six weeks in the helmet. A 47-year-old former elementary-school teacher in the Houston area, she spent two decades “on and off of every antidepressant,” she told me. She has been diagnosed with bipolar II, and she also has generalized anxiety disorder, PTSD, and panic attacks. The anxiety has led her to develop agoraphobia. “I don’t leave my house unless I absolutely have to. It’s just bad,” she said. “Sometimes if I’m in the grocery store, I will have to leave my cart and just go home.”
Tucker has also been suicidal. “The suicidal thoughts were so severe that I never told anyone,” she told me, “not my doctor, not my husband. Because when you tell someone, then they’ll try to save you.”
A few years ago, she began seeing a new psychiatrist who had a video commercial for the NeuroStar TMS helmet playing on a loop in his waiting room. (NeuroStar provides all marketing material for patients. Clinicians buy the machine, and they also pay for each use of it.) She was persuaded to try it. She was “tapped,” as they call it, “on the left side for depression, and on the right side for anxiety.” She sent me a video from her phone of her undergoing treatment. Her face is expressionless, and the piercing blasts sound like laser guns.
After six weeks of daily treatment, Tucker saw no improvement. “I would love to say it worked, but I felt no different when it was over,” she said. “I’m still depressed as fuck.”
One thing that did help, though, was that Tucker made friends with her technician, Allison Rose Zartier, over the course of weeks of treatment. “Having someone like Allison administer TMS was actually the best part for me,” Tucker said. Zartier, who is now the TMS coordinator at a business called Elite Medical Wellness, in Lake Charles, Louisiana, told me she finds it unbelievable that some practitioners leave people alone during the 20-to-30-minute treatments. Some of the biggest benefits she has seen have come through talking with people while the magnets are firing. She said that a recently retired CEO needed to find a way to have purpose with all his free time. Another extremely isolated person felt better after Zartier recommended getting a dog.
Advocates of the depression helmet consider the treatment great if it ends up actually helping even a small number (and harming fewer people than it helps). For ECT and TMS, and anything that proposes to offer a hard restart to your central nervous system, the health risks cannot be zero—and should be expected to be significant. TMS and other high-tech, high-cost treatments also have the potential to divert focus from social, structural, and preventive support—the basic elements of health that, when ignored, often manifest as depressive symptoms.
The medical model of depression tends to offer treatments that imply they can fix emotions that may actually be related to a need to feel valued and secure. Addressing these and other basic imperatives—to sleep and eat well, and be physically active and socially connected—is the first priority for treating and preventing most illnesses, mental and otherwise. This emphasis can be lost when an expensive magnetic helmet that promises to make the feelings somehow simply go away is seen as anything other than a last resort.
Zartier went through TMS herself, and told me she was able to stop taking antidepressants shortly after. She now runs a Facebook support group for TMS patients, in which Hushaw is also active. Zartier said the shared experience of having gone through this process fosters a sense of community. It’s also a tool for recruiting prospective patients. Zartier said the $12,000 cost can be well worth it. She tells people it’s like “going to the gym, but for your brain.”
That community is growing. “It keeps climbing faster, especially in the last four months. The word is finally getting out there,” Zartier told me, which she believes is partly because NeuroStar is now running television commercials. And she’s seeing more and more parents in the concerned Facebook community bringing in children.
“I had a 10th grader who was suicidal, and I saw the pain in her eyes,” she said. “The younger you are, the more the brain can be affected—their brains seem to want to change.”