Mechanism discovered that helps viruses like monkeypox to block and evade our cellular defense system
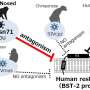
A defense mechanism that human cells possess against viruses such as monkeypox, herpes simplex and human papillomavirus—all double-stranded DNA viruses—relies on proteins that patrol the cell, acting as sensors of the virus's DNA. This type of cellular defense was discovered only a decade ago and is still little studied. When the sensor proteins detect viral DNA they bind to it and the alarm is raised, activating the cell's defenses. But as in any arms race, some viruses have also evolved proteins capable of blocking this cellular alarm system.
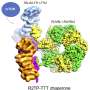
One of these proteins that alerts cells to the presence of DNA viruses is the protein complex called Ku. Scientists at the CNIO and the University of Sussex have now managed to determine its three-dimensional structure at the atomic level, coupled with that of the viral proteins capable of blocking its functioning. The findings, published in the journal Nature Communications, should help to improve the response to these types of infections.
Researchers worked with the Vaccinia virus (used in the development of the smallpox vaccine and belonging to the poxvirus family). Two proteins in this virus, called C4 and C16, bind to Ku and block its action, thereby inactivating the cellular immune response. Knowing the shape of these proteins—their three-dimensional structure—helps researchers to understand how they block Ku.
Proteins as plugs that inactivate the Ku ring
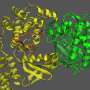
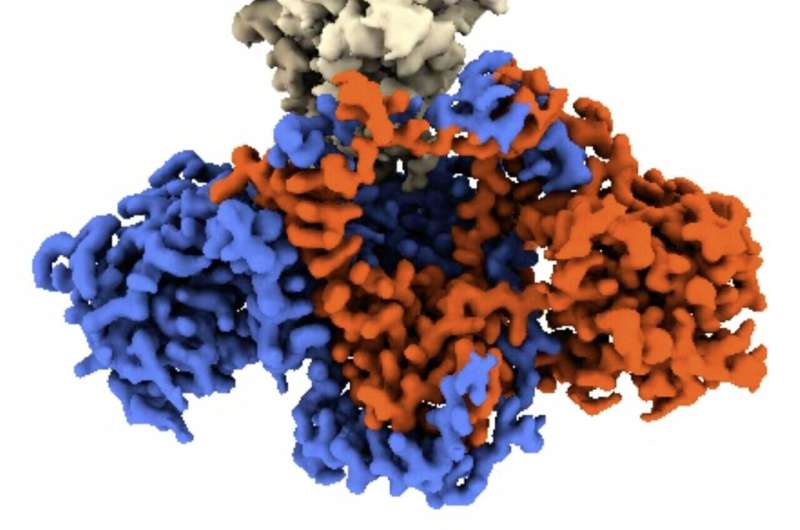
Ku is shaped like a ring, with a central hole that it uses to thread itself into DNA. Researchers have discovered that the two proteins in the virus act as plugs that block the hole, inhibiting Ku's ability to recognize viral DNA.
CNIO researchers from the Macromolecular Complexes in DNA Damage Response Group, led by Oscar Llorca, have succeeded in obtaining the structure of the C16-Ku complex using electron cryomicroscopy, a technique that allows the interactions between the viral protein and the human protein to be visualized.
In this way, the authors of the study have been able to identify which part of the viral protein blocks the functioning of Ku. "The Ku heterodimer forms a kind of ring that binds to the DNA. The virus protein acts as a sort of cap on this ring, blocking the binding of Ku to the viral DNA," explains Oscar Llorca.
The work has been carried out in collaboration with the group at the University of Sussex (UK) led by researcher Laurence H. Pearl, who has confirmed that the mechanism of action of the C4 protein is very similar to that of C16.
Blocking Ku from helping tumor cells
The Ku complex is also present in the nucleus of cells, but its role there is not to alert us to the presence of viruses but to repair our own genetic material when it is damaged.
Llorca's group studies the role in cancer of protein complexes such as Ku, which are involved in the repair of double-stranded DNA. When these repair mechanisms act in tumor cells, they increase their survivability and thus help the cancer. Thanks to this new study, researchers now know how viruses block the action of Ku, which could teach them how to alter its role in repairing DNA breaks in tumor cells.
"The idea for this research came about because if, as part of a treatment to generate DNA damage in tumor cells, we could block Ku from functioning during the DNA repair process, similar to the way viruses do, the treatment would be even more effective," says Angel Rivera-Calzada, co-lead author of the study.
One of the next steps will therefore be to assess whether emulating the mechanism used by viral proteins to block Ku could be utilized to develop a strategy to amplify the effect of cancer treatments.
"Of the entire viral protein, a portion of just a few amino acids acts to block the action of Ku," says Rivera-Calzada. The first step would be to confirm that these small fragments produced in the lab are capable of blocking the recognition of damaged DNA. To achieve this, the authors of the study hope to collaborate with CNIO experts on such strategies.
Furthering the goal of combating viral infections
In addition, the information obtained could help in the development of strategies against infections caused by these viruses.
By comparing the protein sequences of the C4 and C16 homologues in other viruses of the same family, the researchers have been able to observe that the regions involved in Ku inactivation are widely conserved. These viruses include, for example, smallpox and monkeypox, the latter of which has recently been in the news due to the appearance of multiple cases in several European countries. To this end, the researchers are planning future collaborations with groups specializing in virology.
More information: Angel Rivera-Calzada et al, Structural basis for the inactivation of cytosolic DNA sensing by the vaccinia virus, Nature Communications (2022). DOI: 10.1038/s41467-022-34843-z
Journal information: Nature Communications
Provided by The Spanish National Cancer Research Centre