Researchers find fat burning molecule in mice
Linked to serious health problems including cancer, diabetes and cardiovascular disease, obesity affects more than a third of adults in the United States. Presently, there are few safe and effective nonsurgical therapeutic interventions available to patients with obesity.
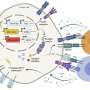
Now, a multi-disciplinary team of researchers has demonstrated that a metabolic regulatory molecule called Them1 prevents fat burning in cells by blocking access to their fuel source. Led by microscopy experts at Beth Israel Deaconess Medical Center (BIDMC) and metabolism experts at Weill Cornell Medicine and NewYork-Presbyterian, the study may contribute to the development of a new type of obesity treatment. The team's findings were published June 9 in Nature Communications.
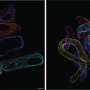
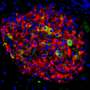
To help explain how the protein Them1 turns off heat production, BIDMC's cell biology and microscopy expert, Susan Hagen, Ph.D., associate vice-chair for research in the Department of Surgery at BIDMC, and Yue Li, Ph.D., a postdoctoral researcher in her laboratory, used light and electron microscopy to observe Them1 in action in mouse brown fat cells grown in the laboratory.
"Them1 is an interesting molecule," said Hagen. "If you inhibit or block its expression, metabolism increases and that reduces body weight."
The experiments showed that when the cells are stimulated to burn fat, a chemical modification causes Them1 molecules to spread out, or diffuse, throughout the cell. This frees the cellular powerhouses called mitochondria to efficiently turn the cell's fat stores into energy. But when the stimulation stops, Them1 molecules quickly reorganize into a structure called a biomolecular condensate. Situated between the mitochondria and the fats they use as fuel, the condensed Them1 molecules limit energy production.
"It turned out to be so incredibly interesting," said Hagen, who is also director of Microscopy and Histology Core Facilities at BIDMC and associate professor of surgery at Harvard Medical School. "We asked other microscopy experts whether they had ever seen anything like the unusual images we found in resting cells. Using very sophisticated electron microscopy techniques, we were able to show—for the first time, as far as we know—what the bimolecular condensate looks like in electron microscopy."
"The study explains a new mechanism that regulates metabolism," said David Cohen, chief of the Division of Gastroenterology and Hepatology at Weill Cornell Medicine and NewYork-Presbyterian/Weill Cornell Medical Center and the Vincent Astor Distinguished Professor of Medicine at Weill Cornell Medicine. "Them1 hacks the energy pipeline and cuts off the fuel supply to the energy-burning mitochondria. Humans also have brown fat and produce more Them1 in cold conditions, so the findings may have exciting implications for the treatment of obesity."
Cohen and Hagen, both members of the Harvard Digestive Diseases Center, have been collaborators since 1983. The current study—supported in part by a five-year, multi-PI grant from the National Institutes of Health—also included collaborators with expertise in structural biology from Emory University.
"This was the most fun I have ever had in science in my life," Hagen added. "Including multiple primary investigators with different expertise gives you the power of doing things that you could never do on your own."
More information: Yue Li et al, Thioesterase superfamily member 1 undergoes stimulus-coupled conformational reorganization to regulate metabolism in mice, Nature Communications (2021). DOI: 10.1038/s41467-021-23595-x
Journal information: Nature Communications
Provided by Beth Israel Deaconess Medical Center