Artificial cells can glow at room temperature in response to external target molecules
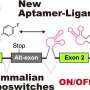
![Construction of theophylline-responsive, eukaryotic kinetic ON-riboswitches with a highly efficient chimeric IRES between the PSIV and CrPV[GCA] IRESes. Credit: ACS Synthetic Biology (2024). DOI: 10.1021/acssynbio.4c00696 Artificial cells that glow at room temperature in response to external target molecules](https://scx1.b-cdn.net/csz/news/800a/2025/artificial-cells-that.jpg)
Cell-free systems, which can express an easily detectable protein with a DNA or mRNA template without constraints of living cells, are attractive as foundations for biosensors. Moreover, by encapsulating them in lipid bilayer membranes (such as liposomes) like natural cells, these systems can avoid the adverse effects of surrounding expression inhibitors.
In order for a cell-free system-based sensor to recognize a target molecule and subsequently express a reporter protein (e.g. glowing protein), target-to-protein signal transduction must be introduced. A promising candidate for this purpose is a riboswitch, a molecule-responsive gene-regulatory sequence. When it is fused to a reporter protein gene, it can regulate (repress or promote) the protein expression in response to a specific molecule.
Although the diversity of natural riboswitches (or that of their target molecules) is limited, a riboswitch responsive to a user-defined molecule can be artificially created. In fact, some natural or synthetic upregulating riboswitches (each fused to a reporter gene) have been used with cell-free systems to create artificial cell-based sensors for detecting membrane-permeable targets.
However, all such sensors reported to date are based on prokaryotic cell-free systems and therefore do not function well at room temperature. There are also problems with riboswitch design that make it difficult to expand the variety of target molecules.
Published in ACS Synthetic Biology, the researchers thus utilized a eukaryotic cell-free system (wheat germ extract), which functions over a wide range of room temperature, and a highly modular synthetic riboswitch (upregulating one) that functions efficiently there. For the latter, they changed the target specificity simply by replacing the target recognition domain in the riboswitch based on their unique rational design method.
In fact, they created three types of synthetic riboswitches (each for one of three target drugs) and fused each to the corresponding gene encoding a reporter protein (green, red, or blue glowing protein). The researchers then encapsulated one of the fusions with wheat germ extract in natural cell-sized liposomes to create artificial cells, each glowing with an intensity dependent on the concentration of its target outside and a color specific to the target.
In addition, due to their high orthogonality, a cocktail of these artificial cells allowed for simultaneous detection of the three targets at room temperature.
More information: Hajime Takahashi et al, Simultaneous Detection of Multiple Analytes at Ambient Temperature Using Eukaryotic Artificial Cells with Modular and Robust Synthetic Riboswitches, ACS Synthetic Biology (2024). DOI: 10.1021/acssynbio.4c00696
Journal information: ACS Synthetic Biology
Provided by Ehime University